
Climate Change Knowledge
Carbon dioxide
Carbon dioxide is a greenhouse gas molecule by virtue of its atomic structure of 3 atoms that gives it the property of the dipole effect. Because of the dipole CO2 absorbs and is energized by infra red heat radiation emanating surface of the planet, resulting in the GHG CO2 molecule radiating infra red heat energy itself. This warms the lower atmosphere and so the planet's surface.
CO2 is a trace atmospheric gas present in only parts per million (parts of air). As the presence of the trace GHGs causes the temperature of Earth to be 30C hotter than without them the trace GHGs like CO2 are very powerful heat radiators in the atmosphere.
In the first hundred years CO2 is absorbed into the upper ocean. The resulting
acidification limits further uptake by the upper ocean waters. During this time period, there is also typically some uptake by the land biosphere. In the next 900 years, the saturated upper ocean waters mix with the deep ocean, allowing further uptake. Eventually, the deep ocean acidifies as well, limiting further uptake. Over the next 10,000 years the ocean becomes buffered by dissolution of carbonate sediments and by carbonates washed in from land, reducing the acidity and allowing the ocean to take up additional carbon. Over longer time scales spanning more than 100,000 years, most of the remaining CO2 is removed by reacting with silicate minerals to form carbonates (e.g., limestone). NRC 2010.

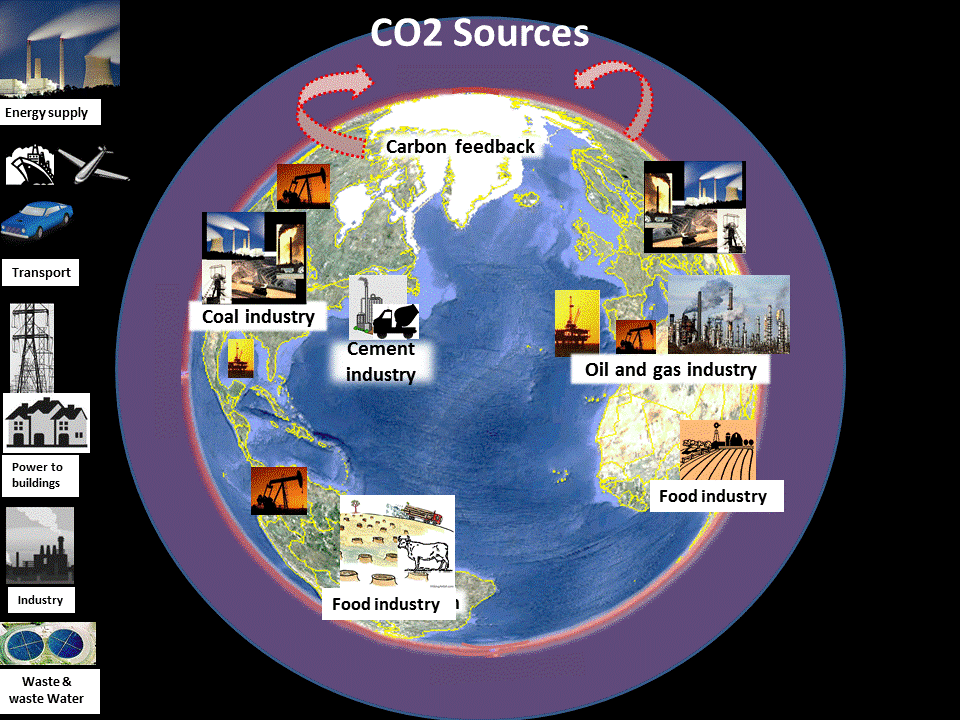
The standard sectoral categories of CO2 emissions are shown at the top of the page. The Earth image here shows a simpler more policy practical breakdown as industrial sources. Also shown is carbon feedback as a source of more carbon emissions (from the global warming of industrial emissions).
Most important about CO2.
CO2 is a highly persistent and so highly accumulative in the atmosphere.
The reason is it lasts- like forever. 1000 years after a CO2 emission 20-40% is still in the atmosphere- heating the surface and acidifying the oceans. It takes 100,000 years for all the CO2 to be removed from the atmosphere as fossil carbon (IPCC AR5)..
CO2 is a highly persistent and so highly accumulative in the atmosphere.
The reason is it lasts- like forever. 1000 years after a CO2 emission 20-40% is still in the atmosphere- heating the surface and acidifying the oceans. It takes 100,000 years for all the CO2 to be removed from the atmosphere as fossil carbon (IPCC AR5)..
The science of CO2 is the science of the carbon cycles. Yes cycles- the very long term carbon cycle is the one that matters most to global warming and climate change and ocean acidification.
Dual polluting effects-
- atmospheric warming
- ocean acidifcation (as well as warming)
Atmosphere.
- Atmospheric CO2 cannot drop or even stabilize unless no more CO2 is added- zero carbon emissions.
- This is because of the very long time of CO2 in the atmosphere and extreme long atmospheric life time of a large proportion of emitted CO2, which is due to the fact that natural processes to sink CO2 (remove CO2 from the carbon cycle) are ultra slow.
- This makes CO2 emissions highly persistent (see computer model result for decay of CO2 emissions) and so cumulative in the atmosphere.
- Any more CO2 added will increase the atmospheric concentration.
- Global warming caused by increased atmopsheric CO2 will last as long as the additional CO2 which is thousands of years at least.
Atmospheric CO2 concentration is unprecedented.
- CO2 is the highest in 15 million years by ocean sediment research.
- It is the highest in 800,000 years by ice cores
- It is increasing at an exponential rate (accelerating rate of increase).
- Ice cores show that CO2 is being added to the atmosphere 14,000 times faster than natural processes can remove it.
- Therefore emissions will continue to increase the rate of increase.
- The increased atmospheric CO2 will last over a thousand years.
Oceans (CO2 dissolves in water to carbonic acid).
- Ocean acidicification is the three times higher than it has been for 20 million years.
- The rate of acidfication is 100 times faster than the past 20 million years.

CO2 and CO2 equivalents.
The term CO2 equivalent is used to combine the main GHGs together.
The term “carbon dioxide equivalents” is used in two main ways for climate change stabilization targets:
• Some authors use “carbon dioxide equivalents” to refer to the concentration of carbon dioxide that would give the same warming effect as the collective effect of all of the greenhouse gases in the atmosphere. This approach excludes the cooling effect of aerosols (e.g. Stern 2007; Garnaut 2008).
• Some authors define carbon dioxide equivalent concentrations as the net forcing of all anthropogenic radiative forcing agents including greenhouse gases, tropospheric ozone, and aerosols but not natural forcings. This approach includes the cooling effects of aerosols (e.g. Meinshausen et al 2006).
The term CO2 equivalent is used to combine the main GHGs together.
The term “carbon dioxide equivalents” is used in two main ways for climate change stabilization targets:
• Some authors use “carbon dioxide equivalents” to refer to the concentration of carbon dioxide that would give the same warming effect as the collective effect of all of the greenhouse gases in the atmosphere. This approach excludes the cooling effect of aerosols (e.g. Stern 2007; Garnaut 2008).
• Some authors define carbon dioxide equivalent concentrations as the net forcing of all anthropogenic radiative forcing agents including greenhouse gases, tropospheric ozone, and aerosols but not natural forcings. This approach includes the cooling effects of aerosols (e.g. Meinshausen et al 2006).
